What is Silicon dioxide?
It is obtained as a transparent to grey, in its crystalline or amorphous powdered form. It is odourless and tasteless compound.
Table of Contents
Recommended Videos
Properties of Silicon dioxide - SiO2
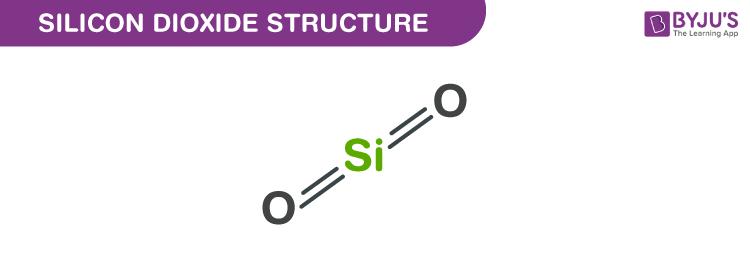
Silicon dioxide Structure - SiO2
SiO2Uses (Silicon dioxide)
Production of Silicon dioxide
Amorphous silica or precipitated silica is obtained by the acidification of sodium silicate solutions. Silica gel is washed and dehydrated to produce colourless microporous silica. The reaction involving a trisilicate along with sulphuric acid is given below:Na2Si3O7 + H2SO4 → 3SiO2 + Na2SO4 + H2O
Silicon dioxide Reactions
SiO2 + 6HF → H2SiF6 + 2H2O
Health hazards
Silica when ingested orally is non-toxic. As per a study conducted in the year 2008, found that the higher the levels of silica in water, the risk of dementia decreased. Therefore, the dose was increased to 10 mg/day of silica in drinking water as the risk of dementia decreased. When finely divided crystalline silica dust is inhaled, it can lead to bronchitis, lung cancer, or silicosis, due to the lodging of dust in the lungs. When fine silica particles are inhaled in large enough quantities, it increases the risk of rheumatoid arthritis and lupus. Learn more about the Structure, physical and chemical properties of SiO2 from the experts at BYJU’S.
Bạn đã thích câu chuyện này ?
Hãy chia sẻ bằng cách nhấn vào nút bên trên
Truy cập trang web của chúng tôi và xem tất cả các bài viết khác!