Potassium tert-Butoxide (KOt-Bu) Is A Bulky Base
In a blatant plug for the Reagent Guide and the Reagents App for iPhone, each Friday I profile a different reagent that is commonly encountered in Org 1/ Org 2.
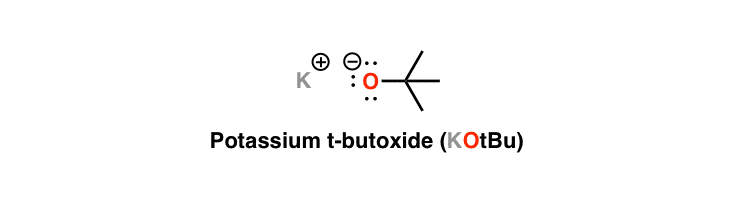
Sometime back in general chemistry you (hopefully) learned that hydroxide ion (HO-) is a strong base. It’s the conjugate base of water - that is to say, that’s what’s left behind once we’ve ripped a proton (H+) off of it. Likewise, alcohols (ROH) are strong bases too - once you remove the proton to get the conjugate base (RO-). Similar in strength to the hydroxide ion, these are called alkoxides.
Potassium tert-Butoxide (KOt-Bu) Is A Bulky Base
Today’s reagent, potassium tert-butoxide (KOt-Bu), is a strong base just like all alkoxides, but there’s something about it that makes it special. If you’ve come across the tert-butyl group before, you should be able to remember one main thing: it’s really darn fat (although in these more sensitive times, “bulky” is the preferred nomenclature). And as the conjugate base of t-butanol, that makes t-butoxide a bulky base just like our old friend LDA. (Note that the “potassium” isn’t so crucial here and we can leave it out or replace it with sodium or lithium- it’s really the “t-butoxide” part we really care about.)
Potassium t-butoxide is like a really angry Sumo wrestler. It attacks things that are out in the open with fierce and sturdy determination. However, anything that requires the least bit of navigation through a narrow opening (like a doorway) is going to be difficult. In the chemical sense, this means that tert-butoxide is very sensitive to steric interactions. (What are “steric interactions” ? Think about 4 hungry Sumos trying to fit themselves around your tiny dinner table). More specifically, steric interactions are the repulsive interactions between electron clouds that happens when atoms “bump” into each other.
Potassium tert-Butoxide Is A Poorer Nucleophile Than Other Alkoxides Due To Steric Hindrance
So what does this mean for the chemical reactions of t-butoxide? Two things.
First, tert-butoxide is a poorer nucleophile than smaller alkoxides (like ethoxide, methoxide and so on) in nucleophilic substitution reactions (like the SN2). Why? Because the SN2 is very sensitive to steric interactions, and tert-butoxide is bulky.
As A Base, tert-Butoxide Tends To Favor The “Non-Zaitsev” of “Hofmann” Product In Elimination Reactions
- tert-butoxide can be used to form the “less substituted” alkenes in elimination reactions (the E2, specifically). Most of the time, elimination reactions favor the “more substituted” alkene - that is, the Zaitsev product. However, when tert-butoxide is used, it will preferentially remove the proton from the smaller group. This produces the so-called “Hoffmann” product. Let’s have a look.



